Reduce reporting time. Restore your department's capacity.
Raygenic streamlines radiology reporting and speeds up clinical decision-making. Less manual work, fewer delays, and a calmer team with full visibility and control over the reporting workflow
The Challenge
In high-volume radiology departments, delays rarely start at the scanner. The bottleneck usually appears between the scan and the final clinical decision. Backlogs grow, radiologists work under constant pressure, and urgent cases compete with routine studies in the same queue
In high-volume radiology departments, delays rarely start at the scanner. The bottleneck usually appears between the scan and the final clinical decision. Backlogs grow, radiologists work under constant pressure, and urgent cases compete with routine studies in the same queue
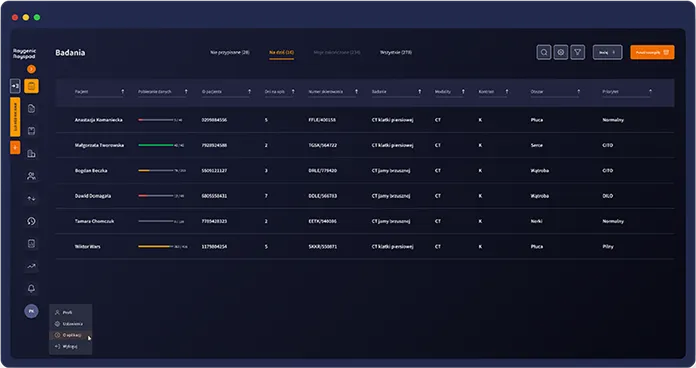
All exams in one queue, limited prioritisation
Manual measurements and reporting slow down throughput
Radiologists switch between multiple systems during reporting
Limited visibility of workload, case status, and reporting progress
Delays increase stress and add risk in time-sensitive cases
Urgent cases can be prioritised immediately
Measurements and reporting steps are supported with automation and AI insights
Exams, annotations, and reports are handled in one workspace
Clear visibility of case status and team workflow
More predictable reporting process with fewer delays
Who is it for?
Raygenic works best in:
Hospitals with high imaging volumes
MRI and CT departments
Diagnostic imaging centres
Facilities struggling with reporting backlogs
Radiology teams needing faster clinical decision-making and better workflow control
Hospitals with high imaging volumes
MRI and CT departments
Diagnostic imaging centres
Facilities struggling with reporting backlogs
Radiology teams needing faster clinical decision-making and better workflow control
Clinically validated. Certified. Built for real hospital conditions
Raygenic is a CE-certified medical device developed with clinicians and tested in university hospital environments.
How Raygenic works
One integrated workspace for exam review and structured reporting
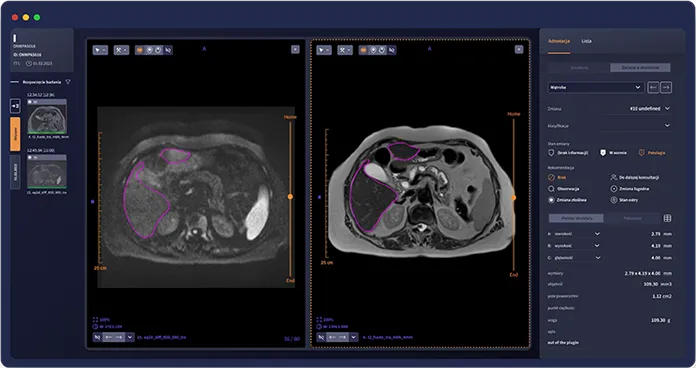
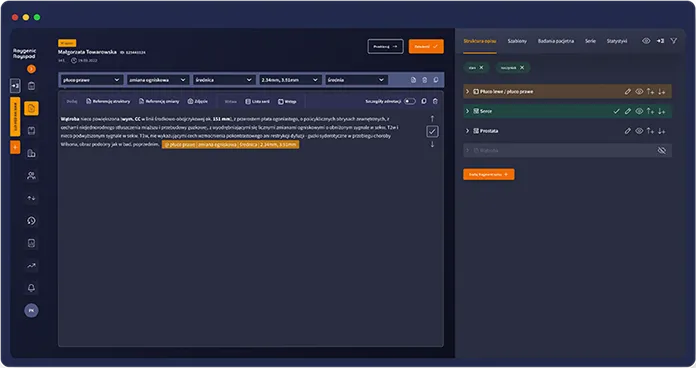
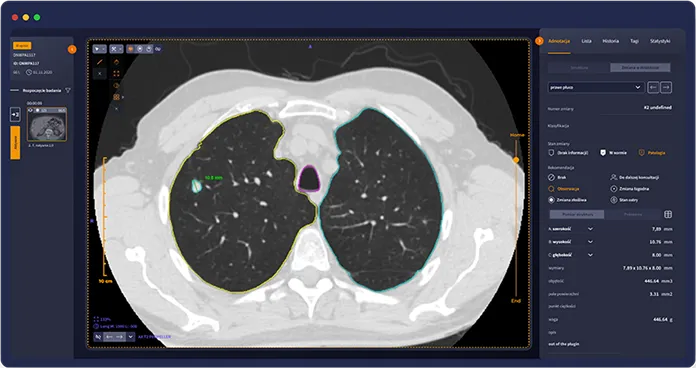
Faster clinical decisions
Shorten the path from scan to clinical decision, especially in backlog-heavy departments.
Shorter reporting backlogs
Increase throughput by removing workflow bottlenecks in reporting.
Less manual work for radiologists
Reduce repetitive steps with automation and AI support.
Lower risk of oversight
AI insights and structured workflow help maintain quality under time pressure.
How collaboration works?
Clear steps that take you from initial discussion to a working solution
Conversation & demo
We show how Raygenic fits your radiology workflow and where reporting time is being lost.
Integration & configuration
Raygenic connects with your PACS, RIS and HIS. We configure workflows and access.
Go-live & support
Raygenic runs in the browser. After go-live, your team works in one environment, with ongoing support.
FAQ
Common questions, clearly answered
Yes. Raygenic is a CE-certified medical device (MDR, class IIa), tested in Polish university hospitals and built together with clinicians.
Raygenic improves workflow between scan and clinical decision through prioritisation, reduced manual steps, and full case visibility in one reporting environment.
Yes. OnTax keeps a full action log showing who uploaded, generated and submitted each file. It also stores confirmations and change history, so audit preparation does not depend on manual reconstruction.
Raygenic reduces system switching and manual work by bringing exams, AI insights, and reporting into one workspace.
Facilities typically reduce reporting backlogs and improve throughput. Many recover their investment within months through improved capacity utilisation.